
Redox reactions are chemical reactions that involve the transfer of electrons between two species. They are an important type of chemical reaction because they are involved in many biological processes, as well as in industrial processes such as the production of metals. Understanding redox reactions is essential for understanding chemistry as a whole.
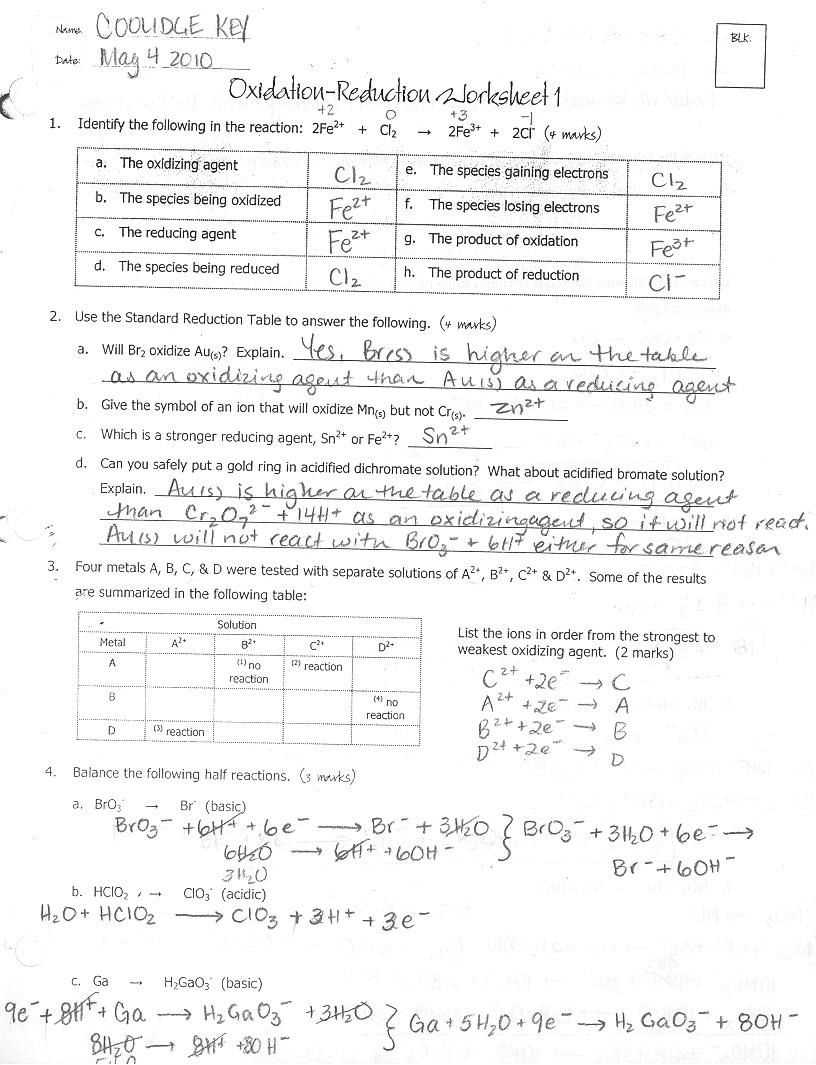
One way to practice and test your understanding of redox reactions is through the use of worksheets. These worksheets typically contain a series of questions and problems that require you to balance redox equations, identify the oxidizing and reducing agents, determine the oxidation number of different elements, and calculate the change in oxidation number.
Answer keys for redox reactions worksheets provide the correct answers and solutions to the problems and questions on the worksheet. They serve as a valuable resource for students to check their work, review concepts, and learn from their mistakes. Answer keys also allow students to compare their answers with the correct answers and understand where they made errors, helping them to improve their understanding and problem-solving skills.
What are redox reactions?
Redox reactions, also known as oxidation-reduction reactions, are chemical reactions that involve the transfer of electrons between species. These reactions play a vital role in various natural and industrial processes, such as respiration, photosynthesis, and the corrosion of metals. Understanding redox reactions is essential in many fields of science, including chemistry, biochemistry, and environmental science.
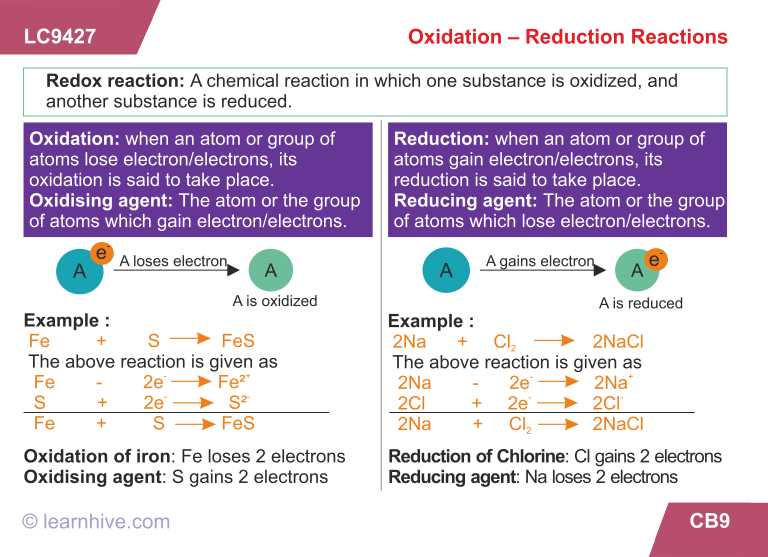
In a redox reaction, one species undergoes oxidation while another species undergoes reduction. Oxidation is the loss of electrons or an increase in oxidation state, while reduction is the gain of electrons or a decrease in oxidation state. These reactions always occur simultaneously because the electrons that are lost during oxidation must be gained by another species during reduction.
Redox reactions are commonly represented using half equations, where the species that is oxidized is shown with its electrons on the left side of the equation, and the species that is reduced is shown with its electrons on the right side. The overall reaction is then balanced by equalizing the number of electrons transferred in both half reactions.
Redox reactions are crucial for maintaining the balance of electrons and energy in biological systems. For example, during cellular respiration, glucose is oxidized to produce energy, while oxygen is reduced to form water. This process allows living organisms to convert the chemical energy stored in food into a usable form.
In conclusion, redox reactions are important chemical processes that involve the transfer of electrons between species. They are essential for various natural and industrial processes and have significant implications in biological systems. Understanding redox reactions is crucial for advancing our knowledge in numerous scientific disciplines and for the development of new technologies.
Key concepts for understanding redox reactions
Redox reactions, short for reduction-oxidation reactions, are fundamental chemical processes that involve the transfer of electrons between species. In these reactions, one substance loses electrons (oxidation) while another substance gains electrons (reduction).
Oxidation refers to the process of losing electrons. It is often characterized by an increase in oxidation state or a decrease in the number of bonding electrons. On the other hand, reduction involves gaining electrons and typically leads to a decrease in oxidation state or an increase in the number of bonding electrons.
One way to understand redox reactions is through the concept of oxidation numbers. Oxidation numbers are assigned to atoms in a molecule or ion to indicate their electron distribution. For example, in a neutral molecule, the sum of oxidation numbers is zero, while in an ion, the sum equals the ion’s charge. Changes in oxidation numbers can help identify which species are being oxidized or reduced in a reaction.
Redox reactions can occur in a variety of contexts, including chemical reactions, biological processes, and electrochemical cells. In chemical reactions, redox reactions play a crucial role in balancing the transfer of electrons between reactants and products. In biological systems, redox reactions are involved in energy production, metabolism, and signaling pathways. Electrochemical cells utilize redox reactions to convert chemical energy into electrical energy.
Understanding the key concepts of redox reactions is important for many areas of science, including chemistry, biology, and environmental science. By recognizing the transfer of electrons and changes in oxidation states, scientists can analyze and predict the behavior of chemical reactions and biological processes. The study of redox reactions also has practical applications, such as in the development of batteries, corrosion prevention, and wastewater treatment.
How to Balance Redox Equations: Step-by-Step Guide
Redox equations, also known as oxidation-reduction equations, are chemical equations that involve both oxidation and reduction reactions. Balancing these equations can be a challenging task, but with a step-by-step approach, you can easily achieve balance. Here is a guide to help you balance redox equations:
1. Identify the Oxidation Numbers
The first step in balancing redox equations is to determine the oxidation numbers of all the elements in the equation. Oxidation numbers represent the charge that an atom would have if electrons were transferred completely. The element that loses electrons is oxidized, while the one that gains electrons is reduced.
2. Separate the Equation into Half-Reactions
Next, separate the overall redox equation into two half-reactions – one representing oxidation and the other representing reduction. This step helps you focus on balancing each half-reaction individually before combining them.
3. Balance the Atoms other than Oxygen and Hydrogen
Begin by balancing the atoms in each half-reaction except for oxygen and hydrogen. Start with the elements that are not part of water or hydrogen ions and adjust the coefficients until the number of atoms on each side of the equation is equal.
4. Balance Oxygen Atoms with Water
To balance the number of oxygen atoms, add water molecules to the side of the half-reaction that lacks oxygen. Remember to adjust the coefficients so that the number of water molecules balances the oxygen atoms.
5. Balance Hydrogen Atoms with Hydrogen Ions
Similarly, balance the number of hydrogen atoms by adding hydrogen ions (H+) to the side of the equation that is deficient in hydrogen. Again, adjust the coefficients as needed.
6. Balance the Charge with Electrons

At this stage, the atoms in each half-reaction should be balanced, but the charges may not be equal. To balance the charge, add electrons (e-) to the side that requires them. The number of electrons must be the same for both half-reactions.
7. Multiply the Half-Reactions
If necessary, multiply each half-reaction by an integer to equalize the number of electrons in both reactions. This step ensures that the total number of electrons transferred is the same in both half-reactions.
8. Combine the Half-Reactions
Now that the half-reactions are balanced, combine them by adding them together. The electrons cancel out, and you are left with the balanced redox equation.
By following these step-by-step instructions, you can successfully balance redox equations and understand the transfer of electrons in chemical reactions.
Common types of redox reactions
Redox reactions, also known as oxidation-reduction reactions, are a fundamental part of chemistry. They involve the transfer of electrons between molecules and atoms, resulting in changes in their oxidation states. There are several common types of redox reactions that occur in various chemical processes.
Combustion reactions: Combustion reactions are one of the most well-known types of redox reactions. They involve the reaction of a substance, usually a hydrocarbon or an organic compound, with oxygen gas to produce carbon dioxide and water. This type of reaction releases energy in the form of heat and light.
Displacement reactions: Displacement reactions occur when one element replaces another element in a compound. The element that is being replaced is oxidized, and the element that is replacing it is reduced. One common example of a displacement reaction is the reaction between a metal and an acid, where the metal displaces hydrogen from the acid to form a salt and hydrogen gas.
Reduction reactions: Reduction reactions involve the gain of electrons by a molecule or atom, resulting in a decrease in its oxidation state. These reactions often involve the addition of hydrogen or the removal of oxygen from a compound. An example of a reduction reaction is the conversion of iron(III) oxide to iron metal by reacting it with carbon monoxide gas.
Oxidation reactions: Oxidation reactions involve the loss of electrons by a molecule or atom, resulting in an increase in its oxidation state. These reactions often involve the addition of oxygen or the removal of hydrogen from a compound. A common example of an oxidation reaction is the reaction of an alcohol with an oxidizing agent, such as potassium dichromate, to form an aldehyde or a ketone.
- Another type of redox reaction is a disproportionation reaction, where a single substance is both oxidized and reduced at the same time.
- In addition, redox reactions can also occur in biological systems, such as in cellular respiration where glucose is oxidized to produce carbon dioxide, water, and ATP.
Overall, redox reactions play a crucial role in various chemical processes and have significant implications in fields such as energy production, environmental science, and pharmaceuticals.
Redox reactions in everyday life

In our everyday lives, we encounter redox reactions without even realizing it. Redox reactions play a crucial role in several processes that are essential for our daily activities. Here are some examples:
- Breathing: The process of respiration involves redox reactions. When we inhale, oxygen is reduced, and when we exhale, carbon dioxide is oxidized.
- Metabolism: Redox reactions are involved in the breakdown and synthesis of food molecules in our bodies. The process of converting glucose into energy through cellular respiration is a prime example of a redox reaction.
- Cooking: The process of cooking food involves various redox reactions. For example, when food is heated, the proteins denature, which involves oxidation and reduction processes.
- Metal corrosion: The rusting of iron is a common example of a redox reaction. When iron reacts with oxygen in the presence of moisture, it undergoes oxidation to form iron(III) oxide.
- Batteries: Batteries utilize redox reactions to generate electrical energy. The conversion of chemical energy to electrical energy in batteries involves the movement of electrons between different substances.
Overall, redox reactions are an integral part of our everyday lives, playing a role in essential processes such as breathing, metabolism, cooking, metal corrosion, and even powering our electronic devices through batteries. Understanding redox reactions allows us to appreciate the chemical processes that occur around us and their significance in our daily activities.