In Section 8.3 of the study guide, we explore the properties of acids and bases and how they interact with each other. This section is crucial for understanding the fundamental principles of chemistry and helps us understand the behavior of various substances.
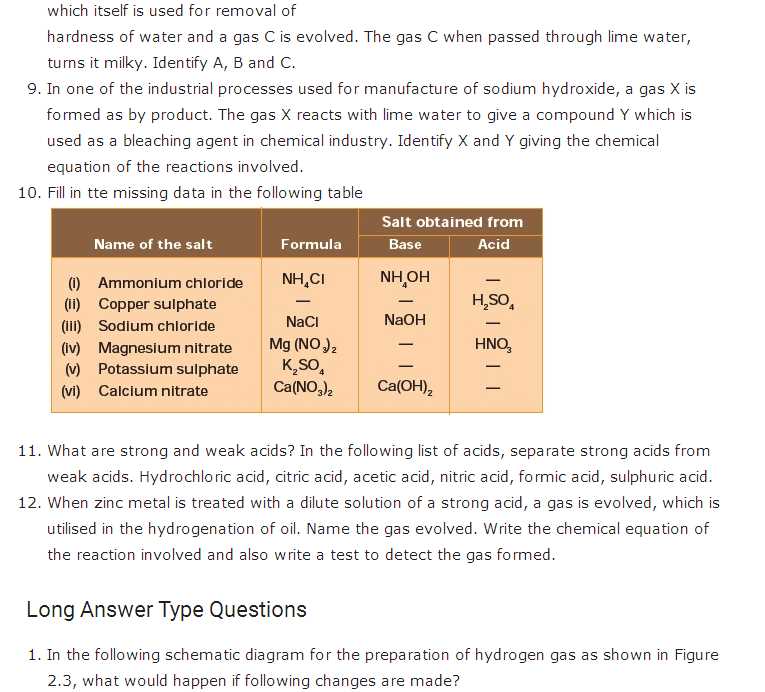
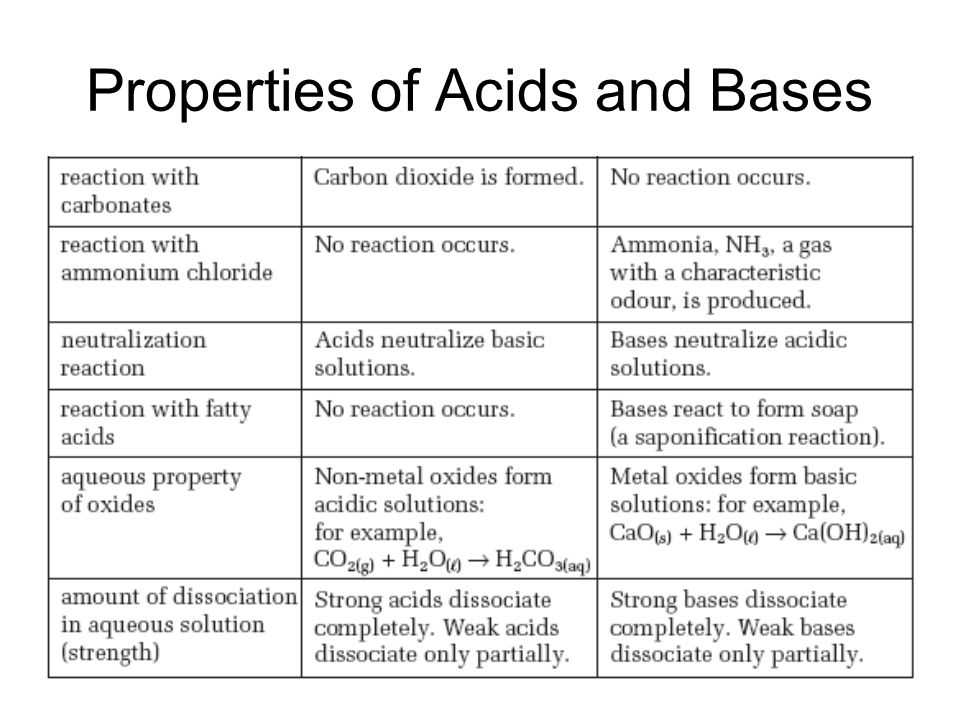
Acids and bases are two types of compounds that play significant roles in chemical reactions. Acids can be identified by their sour taste and ability to turn litmus paper red, while bases taste bitter and turn litmus paper blue. Understanding these characteristics is vital in differentiating between acids and bases and determining their properties.
One of the key properties of acids is their ability to release hydrogen ions (H+) when dissolved in water. This property is referred to as acidity. By contrast, bases release hydroxide ions (OH-) when dissolved in water, giving them their characteristic alkaline properties.
Additionally, acids and bases can react with each other in a chemical reaction known as neutralization. In this reaction, an acid and a base combine to produce salt and water. The neutralization reaction is a crucial concept in chemistry because it helps to understand how acids and bases can counteract each other’s properties.
Properties of Acids and Bases: An Overview
The properties of acids and bases play a crucial role in chemistry and have significant implications in various scientific and everyday applications. Acids and bases are two types of chemical compounds that have distinct characteristics and behaviors. Understanding their properties is essential for predicting their behavior in reactions and for utilizing their unique properties in different industries.
Acids: Acids are substances that release hydrogen ions (H+) when dissolved in water. They have a sour taste and can cause a burning sensation. Acids can react with metals to produce hydrogen gas and with bases to form water and salts. The strength of an acid is determined by its ability to donate protons. Strong acids completely dissociate in water, while weak acids only partially dissociate.
Bases: Bases are substances that release hydroxide ions (OH-) when dissolved in water. They have a bitter taste and a slippery, soapy feel. Bases can react with acids to form water and salts. The strength of a base is determined by its ability to accept protons. Strong bases completely dissociate in water, while weak bases only partially dissociate.
Acids and bases can be classified as strong or weak based on their degree of ionization in water. Strong acids and bases ionize completely, while weak acids and bases only partially ionize. The pH scale is a measure of how acidic or basic a solution is. It ranges from 0 to 14, with 0 being the most acidic, 7 being neutral, and 14 being the most basic.
The properties of acids and bases are utilized in various fields, such as medicine, agriculture, and industry. Acids, such as sulfuric acid, are used in the production of fertilizers, dyes, and pharmaceuticals. Bases, such as sodium hydroxide, are used in the manufacturing of soaps, detergents, and paper. Understanding the properties of acids and bases is essential for ensuring the safety and efficacy of these applications.
Summary:
- Acids release hydrogen ions (H+) and have a sour taste.
- Bases release hydroxide ions (OH-) and have a bitter taste.
- Acids react with metals to produce hydrogen gas and with bases to form water and salts.
- Bases react with acids to form water and salts.
- The pH scale measures the acidity or basicity of a solution.
- The properties of acids and bases are utilized in various industries.
Definition and Classification
An acid is a substance that donates hydrogen ions (H+) in a chemical reaction. It can be classified as a strong acid or a weak acid based on the degree to which it dissociates or ionizes in water. A strong acid fully ionizes in water, releasing all of its hydrogen ions, while a weak acid only partially ionizes, releasing a smaller portion of its hydrogen ions.
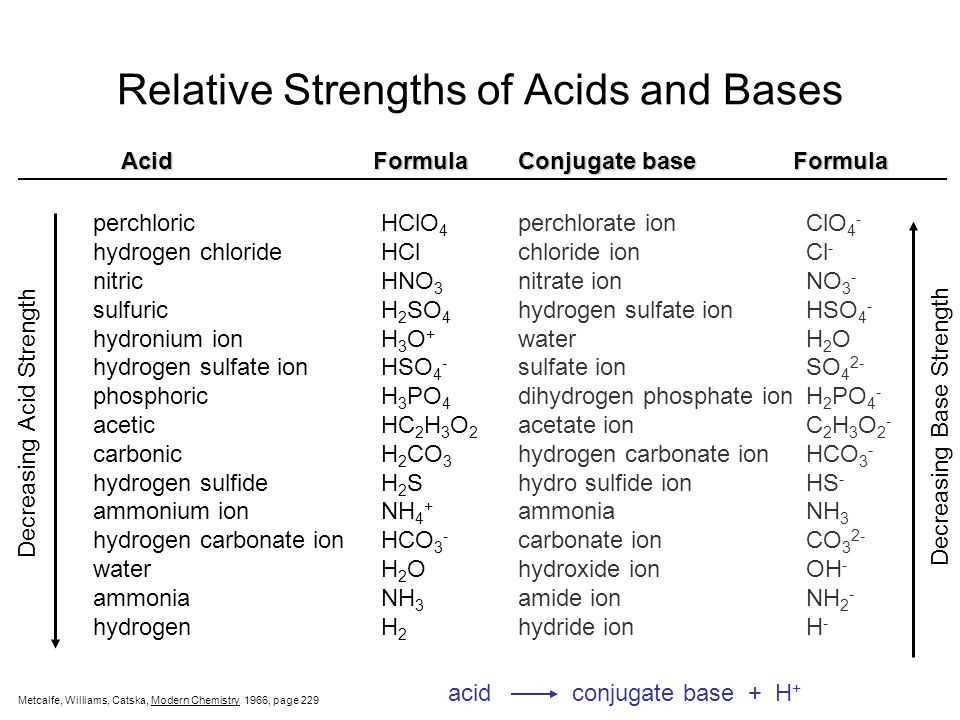
A base, on the other hand, is a substance that accepts hydrogen ions or donates hydroxide ions (OH-) in a chemical reaction. Similar to acids, bases can also be classified as strong bases or weak bases. A strong base fully dissociates in water, releasing all of its hydroxide ions, while a weak base only partially dissociates.
Oxides and hydroxides of metals are generally classified as bases, while oxides and hydroxides of nonmetals can act as acids or bases depending on the specific chemical reaction. Some substances, known as amphoteric substances, have the ability to act as both acids and bases depending on the conditions.
It is important to note that the classification of an acid or base as strong or weak does not necessarily indicate its corrosiveness or harm to living organisms. Strong acids and bases can cause severe burns and tissue damage, while weak acids and bases may still pose a risk in high concentrations or under certain conditions.
Physical Properties of Acids
Acids are a group of chemical compounds that have specific physical properties. These properties distinguish them from other substances and can be used to identify and classify them.
Sour Taste: One of the characteristic properties of acids is their sour taste. This taste is easily recognizable and can be experienced by tasting certain acidic foods, such as lemons or vinegar. However, it is important to note that tasting strong acids can be harmful and should be avoided.
Corrosive: Acids have a corrosive nature, meaning they have the ability to cause damage or destruction to other materials. This property can be observed when acids come into contact with metals, as they can react with them and produce a corrosive effect.
Conductivity: Acids are electrical conductors. When dissolved in water, acids ionize and produce hydrogen ions (H+). These hydrogen ions are responsible for the acidic properties of the solution and allow it to conduct electricity.
pH Level: Acids have a pH value below 7 on the pH scale. The pH scale is a logarithmic scale that measures the concentration of hydrogen ions in a solution. Acids have a higher concentration of hydrogen ions, resulting in a lower pH value.
Reaction with Indicators: Acids can be identified by their reaction with indicators. Indicators are substances that change color in the presence of acids or bases. For example, litmus paper turns red in the presence of an acid.
Corrosive to Organic Matter: Acids have the ability to react with organic matter, causing damage or decomposition. This property is often utilized in industries for purposes such as etching or cleaning surfaces.
In conclusion, acids have several distinct physical properties that set them apart from other substances. These properties, such as their sour taste, corrosive nature, conductivity, pH level, reaction with indicators, and ability to corrode organic matter, are key characteristics used to identify and classify acids.
Chemical Properties of Acids
Acids are a group of chemical substances that have specific properties and behaviors. These properties help us to identify and classify acids, as well as understand their role in various chemical reactions and processes. Let’s take a closer look at some of the key chemical properties of acids:
- Sour taste: One of the most noticeable properties of acids is their sour taste. This property is the reason why substances like lemon juice and vinegar taste sour.
- Reactivity with metals: Acids react with certain metals to produce hydrogen gas. This reaction is known as a single displacement reaction. For example, hydrochloric acid reacts with zinc to produce zinc chloride and hydrogen gas.
- Electrolytic properties: Acids are electrolytes, meaning they can conduct electricity when dissolved in water. The presence of charged particles (ions) allows them to carry an electric current.
- Corrosive nature: Acids have a corrosive nature, meaning they can cause damage to certain materials such as metals, skin, and fabrics. This property is often utilized in industries for various purposes like etching, cleaning, and manufacturing.
- Neutralization reactions: Acids can react with bases to form salts and water in what is known as a neutralization reaction. This reaction helps to balance the pH levels and reduce the acidity or alkalinity of a solution.
In addition to these properties, acids also have specific chemical formulas and can be classified into different types based on their composition and properties. Understanding the chemical properties of acids is crucial in various scientific fields, including chemistry, biology, and environmental science.
Physical Properties of Bases
Bases, like acids, are an important class of substances with distinct physical properties. These properties can be observed and used to identify and characterize bases. Here are some key physical properties of bases:
- Taste: Bases typically have a bitter taste. This is in contrast to acids, which have a sour taste. However, it is important to note that tasting chemical substances is highly discouraged due to their potential toxicity.
- Touch: Bases can feel slippery or soapy to the touch. This is because they react with the fatty acids present in the oils of our skin, forming soap-like substances called salts.
- Electrical conductivity: Bases are electrolytes, which means they conduct electricity when dissolved in water. This is because they dissociate into positive ions (cations) and negative hydroxide ions (OH-) in solution, allowing the flow of electric current.
- Indicator reactions: Bases can react with indicators, such as litmus paper or phenolphthalein, causing characteristic color changes. For example, bases turn red litmus paper blue and phenolphthalein pink.
- Reaction with acids: Bases neutralize acids through an acid-base reaction, forming salts and water. This reaction is often characterized by the production of heat.
- Solubility: Most bases are soluble in water. However, the solubility of bases varies depending on the specific base and the temperature.
By examining these physical properties, scientists can distinguish bases from other substances and determine their chemical properties. It is important to understand the physical properties of bases in order to safely handle and work with these substances.
Chemical Properties of Bases
A base is a substance that can accept or donate a pair of electrons, and it has specific chemical properties. Understanding these properties is important in identifying and characterizing bases.
1. Reactivity with Acids: Bases exhibit a neutralization reaction when they react with acids. These reactions involve the transfer of protons (H+) from acids to bases, resulting in the formation of a salt and water. The strength of the base determines the extent of the reaction.
2. Corrosiveness: Bases are usually corrosive substances that can cause damage to living tissues and metals. Their ability to dissolve substances and react with organic matter makes them potentially harmful if not handled with care.
3. pH Level: Bases have a pH level above 7, indicating their alkaline nature. The higher the concentration of hydroxide ions (OH-) in a substance, the stronger the base. pH levels of bases can range from slightly basic to highly basic.
4. Formation of Salts: Bases react with acids to form salts. The cation of the base combines with the anion of the acid, resulting in a new compound known as a salt. The properties of the salt depend on the specific base and acid used in the reaction.
5. Amphoteric Nature: Some bases, known as amphoteric substances, can act as both bases and acids. They can accept or donate protons depending on the conditions. This property allows them to play a role in various chemical reactions.
Understanding the chemical properties of bases helps in identifying, characterizing, and utilizing these substances in various industries and applications. It also provides insights into their potential effects on living organisms and the environment, emphasizing the importance of proper handling and disposal.
Acid-Base Indicator and Titration
In the study of acids and bases, one important aspect is determining the strength or concentration of these substances. Two common techniques used are acid-base indicators and titration.
Acid-Base Indicators
Acid-base indicators are substances that change color in the presence of acid or base. They are often used to visually indicate the endpoint of a titration, where the reaction between the acid and base is complete. Examples of commonly used indicators include phenolphthalein, bromothymol blue, and methyl orange. Each indicator has a different pH range over which it changes color, allowing for a wide range of acid-base reactions to be monitored.
These indicators work by undergoing a chemical transformation when exposed to an acidic or basic medium. The transformation leads to a change in the absorption or reflection of light, resulting in a visible color change. By observing the color change, one can determine the pH of the solution and whether it is acidic or basic.
Titration
Titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known solution of a reagent, called the titrant, of known concentration. The reaction between the two solutions is often an acid-base reaction, with the endpoint of the reaction indicated by an acid-base indicator.
In a typical acid-base titration, a solution of known concentration, called the titrant, is slowly added to a solution of unknown concentration, called the analyte. The titrant is added until the indicator changes color, indicating the endpoint of the reaction. At this point, the moles of titrant consumed can be used to calculate the concentration of the analyte.
Summary
Overall, acid-base indicators and titration are important tools in the study of acids and bases. Indicators provide a visual indication of the endpoint of a reaction, while titration allows for the determination of the concentration of a solution. Together, these techniques provide valuable information about the properties and behavior of acids and bases.
Q&A:
What is an acid-base indicator?
An acid-base indicator is a substance that changes its color depending on whether it is in an acidic or basic solution. It allows us to visually determine the pH of a solution.
How does an acid-base indicator work?
An acid-base indicator contains a molecule that can undergo a reversible reaction, changing its color depending on the concentration of hydrogen ions (H+) or hydroxide ions (OH-) in the solution. The color change occurs at a specific pH value, known as the indicator’s transition range.
What are some commonly used acid-base indicators?
Some commonly used acid-base indicators include phenolphthalein, bromothymol blue, methyl orange, and litmus paper. Each of these indicators has a different transition range and is suitable for different pH ranges.
What is titration?
Titration is a laboratory technique used to determine the concentration of a solution. It involves adding a solution of known concentration (the titrant) to a solution of unknown concentration (the analyte) until the reaction between the two is complete, as indicated by a color change from the acid-base indicator.