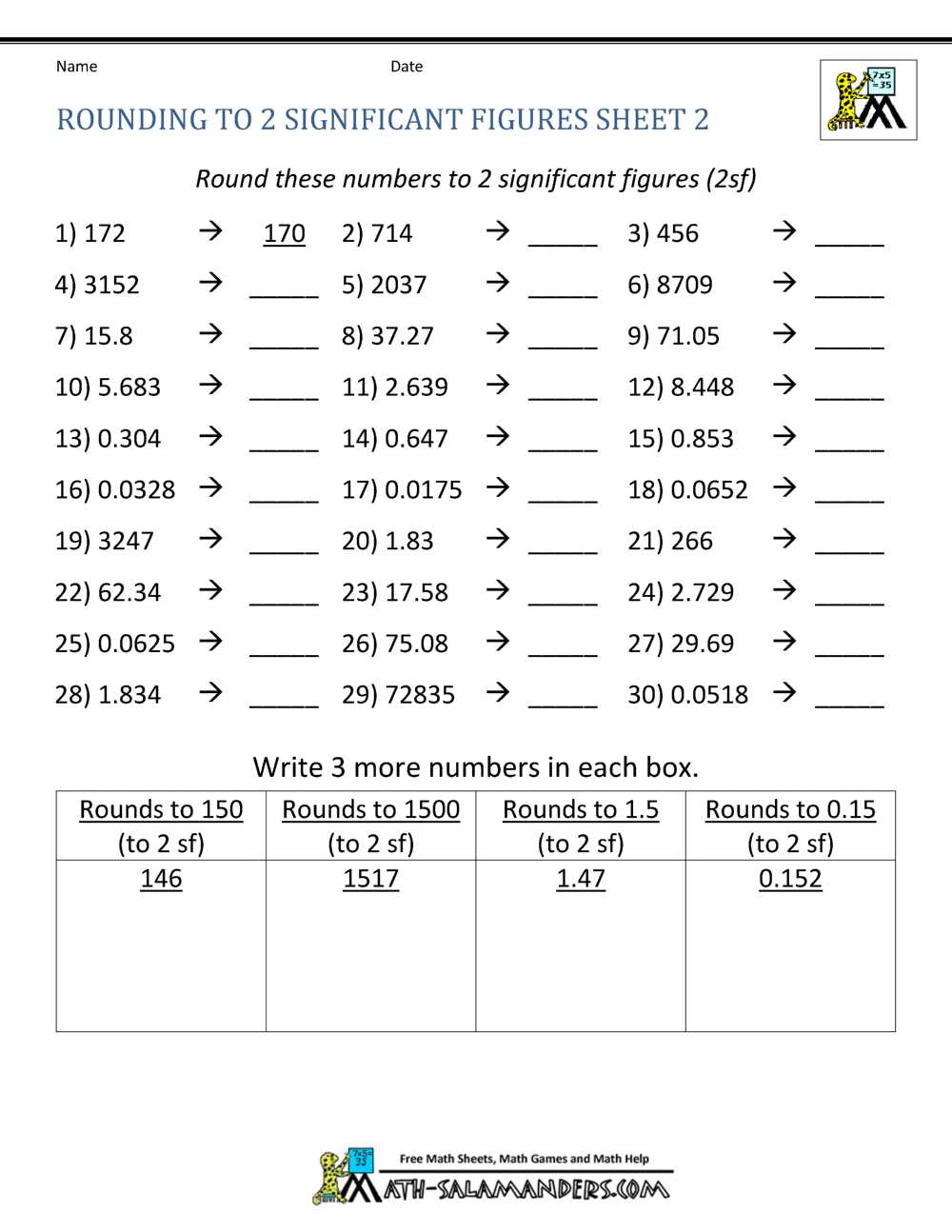
Significant figures, also known as significant digits, are an important concept in mathematics and science. They are used to represent the precision or certainty of a measurement or calculation. The number of significant figures in a value tells us how much information is available and how precise the measurement is. In this practice worksheet, we will review and practice working with significant figures.
This worksheet is designed to help students improve their understanding and skills in identifying significant figures, performing calculations with significant figures, and rounding numbers to the appropriate number of significant figures. It includes a variety of exercises and problems that cover different aspects of working with significant figures.
The answer key is provided at the end of the worksheet, allowing students to check their work and self-assess their progress. By solving these practice problems, students can reinforce their knowledge of significant figures, improve their problem-solving abilities, and gain confidence in their mathematical skills.
Understanding Significant Figures in Chemistry
The concept of significant figures is an important aspect of chemistry, as it helps in determining the accuracy and precision of measurements. Significant figures, also known as significant digits, are the digits in a number that carry meaningful information about the measurement or calculation. In chemistry, significant figures play a crucial role in expressing the uncertainty and limitation of experimental data.
Significant figures are determined based on certain rules. The general rule is that all non-zero digits are significant. For example, in the number 345, all three digits (3, 4, and 5) are significant. In addition, all zeros between non-zero digits are also significant. For example, in the number 3005, all four digits (3, 0, 0, and 5) are significant. However, leading zeros (zeros before the first non-zero digit) are not significant. For example, in the number 0.0021, only the digits 2 and 1 are significant.
Significant figures are also used when performing mathematical operations with measured values. When adding or subtracting numbers, the result should be rounded to the least number of decimal places in the original numbers. For example, if you are adding 24.51 and 16.8, the result should be rounded to two decimal places, giving you 41.31. When multiplying or dividing numbers, the result should be rounded to the least number of significant figures in the original numbers. For example, if you are multiplying 2.41 and 3.69, the result should be rounded to three significant figures, giving you 8.89.
In conclusion, understanding significant figures is crucial in chemistry as it helps in accurately representing experimental data and expressing the uncertainty and limitation of measurements. By following the rules of significant figures, chemists can ensure the accuracy and precision of their calculations and calculations.
What are Significant Figures?

In scientific measurements, significant figures are used to indicate the precision or certainty of a measured value. These figures represent the smallest increment in the measurement that can be reliably determined.
Significant figures are important because they help to express the precision of a measurement and communicate the level of uncertainty in the measurement. They are particularly useful when performing calculations with measurements, as they can help to determine the number of decimal places that should be used in the final result.
The rules for determining the number of significant figures in a measurement are as follows:
- Non-zero digits are always significant. For example, in the number 45.6, there are three significant figures.
- Any zeros between non-zero digits are always significant. For example, in the number 5003, there are four significant figures.
- Leading zeros (zeros that precede non-zero digits) are not significant. For example, in the number 0.0047, there are two significant figures.
- Trailing zeros (zeros that follow non-zero digits) are significant only if they are after a decimal point. For example, in the number 20.00, there are four significant figures.
By following these rules, scientists and researchers can ensure that their measurements are accurate and precise, and that their results are communicated effectively to others in the scientific community.
Importance of Significant Figures in Measurements
Significant figures play a crucial role in measurements across various fields such as chemistry, physics, engineering, and many others. They provide essential information about the precision and accuracy of the measurements and help in ensuring reliable and meaningful data.
Precision: In scientific measurements, it is important to convey the precision of the measured value. The significant figures represent the number of known digits in a measurement plus one estimated digit. This allows scientists and researchers to communicate the level of precision and the potential variability of the measured quantity.
Accuracy: Accuracy refers to how close a measurement is to the true value. Significant figures help ensure accuracy by indicating the level of certainty in a given measurement. By using the appropriate number of significant figures, scientists can avoid misleading or inaccurate conclusions based on measurements with excessive precision.
Rounding and Calculations: Significant figures are also crucial when it comes to performing calculations with measured values. Following the rules of significant figures enables us to retain the appropriate level of precision throughout the calculation. The result of a calculation should be reported with the same number of significant figures as the measurement with the fewest significant figures involved in the calculation.
Reporting Results: Using the correct number of significant figures is important when reporting experimental results. It helps in maintaining transparency and consistency in scientific research and ensures that the findings can be replicated by other researchers. Moreover, it enables the comparison of different measurements and facilitates the identification of trends or patterns.
In conclusion, significant figures are vital tools in scientific measurements as they provide precision, accuracy, and consistency. By following the rules of significant figures, scientists can convey reliable and meaningful information, perform accurate calculations, and report their findings with clarity and transparency.
Rules for Determining Significant Figures
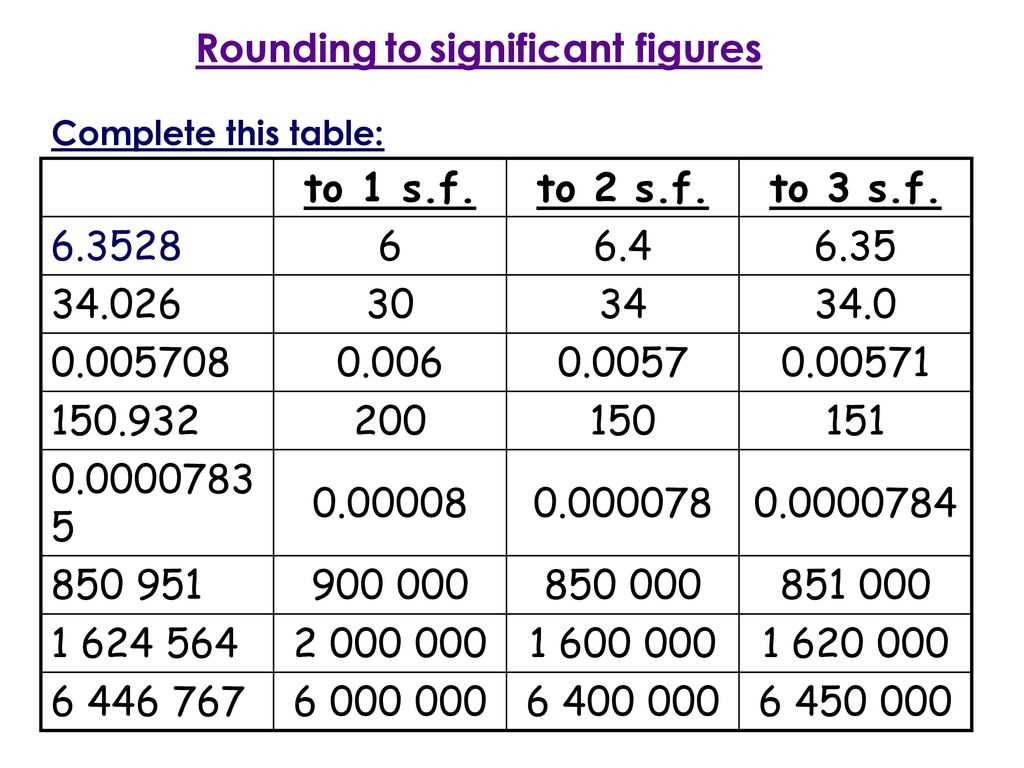
In order to correctly determine the number of significant figures in a given measurement or calculation, it is important to follow certain rules. These rules help to ensure that the number of significant figures is a reflection of the precision and accuracy of the measurement.
1. Non-zero digits are always significant: Any non-zero digit is considered to be significant. For example, in the measurement 12.345 cm, all of the digits (1, 2, 3, 4, and 5) are significant.
2. Zeros between non-zero digits are significant: If a zero is sandwiched between two non-zero digits, it is considered to be significant. For example, in the measurement 102.07 g, there are five significant figures.
3. Leading zeros are not significant: If a zero appears at the beginning of a number, it is not significant. For example, in the measurement 0.0085 L, there are two significant figures.
4. Trailing zeros are significant if they appear after a decimal point: If a zero appears after a decimal point and is at the end of a number, it is considered to be significant. For example, in the measurement 3.00 kg, there are three significant figures.
5. Trailing zeros are not significant if they appear before a decimal point: If a zero appears at the end of a number but before a decimal point, it is not considered to be significant. For example, in the measurement 3000 m, there are only one significant figure.
6. Exact numbers have an unlimited number of significant figures: Exact numbers, such as those obtained from counting or defined values, have an unlimited number of significant figures. For example, if you have 7 apples, the number 7 is considered to have an infinite number of significant figures.
By applying these rules, one can accurately determine the number of significant figures in a given measurement or calculation. Understanding the significance of these figures is crucial in scientific and mathematical calculations, as it allows for more precise and accurate results.
Significant Figures Addition and Subtraction Practice
When performing addition or subtraction with numbers that have different numbers of significant figures, it is important to determine the appropriate number of significant figures in your final answer. This practice will help you develop the skill of determining significant figures in addition and subtraction problems.
To determine the number of significant figures in your final answer, follow these rules:
- When adding or subtracting numbers, your final answer should have the same number of decimal places as the number with the fewest decimal places.
- The final answer should have the same number of significant figures as the number with the fewest significant figures.
Let’s practice with some examples:
| Addition | Subtraction |
|---|---|
| 5.2 + 0.1254 = 5.3254 (round to 5.3) | 8.79 – 2.1 = 6.69 (round to 6.7) |
| 2.345 + 1.0 = 3.345 (round to 3.35) | 12.6 – 1.23 = 11.37 (round to 11.4) |
| 0.004 + 0.0067 = 0.0107 (round to 0.011) | 100 – 58.26 = 41.74 |
Remember to always consider the number of decimal places and significant figures when performing addition and subtraction with significant figures. Practice these rules and you will become proficient in determining the appropriate number of significant figures in your final answer.
Significant Figures Multiplication and Division Practice
When performing calculations with numbers that have significant figures, it is important to correctly determine the number of significant figures in the result. Multiplication and division have rules that govern the number of significant figures in the final answer.
Multiplication: When multiplying numbers, the final answer should have the same number of significant figures as the number with the fewest significant figures. For example, if you multiply 2.5 by 3.456, the final answer should have two significant figures since 2.5 only has two significant figures.
Division: When dividing numbers, the final answer should have the same number of significant figures as the number with the fewest significant figures. For example, if you divide 12.345 by 4.2, the final answer should have two significant figures since 4.2 only has two significant figures.
It is important to keep track of significant figures when performing calculations to maintain the accuracy and precision of the final result. Practice worksheets and exercises can help reinforce these rules and improve proficiency in handling significant figures in multiplication and division. By practicing regularly, you can become more comfortable and confident in working with numbers and determining the correct number of significant figures in the final answer.
Conclusion
The significant figures practice worksheet with answers provides students with an opportunity to practice their skills in identifying and manipulating significant figures. Through a series of exercises, students are able to understand the rules for determining the number of significant figures in a given measurement or calculation.
By practicing with this worksheet, students can improve their proficiency in using significant figures, which is crucial for accurate and precise measurements in scientific calculations. Additionally, the included answers allow students to assess their understanding and correct any misconceptions they may have.
Overall, the significant figures practice worksheet with answers serves as a valuable tool for students to reinforce their knowledge and application of significant figures. With continued practice and understanding, students can confidently apply significant figures to their scientific work and ensure the accuracy and precision of their calculations.