Understanding electrochemical cells is a fundamental concept in chemistry. These cells play a crucial role in powering many of the devices we rely on every day, from batteries in our smartphones to fuel cells in electric vehicles. In order to fully grasp the principles behind electrochemical cells, it is important to have a solid understanding of the various components and processes involved.
The electrochemical cell worksheet provides a comprehensive set of questions and problems to test your knowledge on this topic. It covers a range of concepts, including the basics of oxidation and reduction reactions, the role of electrodes and electrolytes, and the calculation of cell potentials. By working through the worksheet and finding the answers, you can reinforce your understanding of these concepts and boost your problem-solving skills.
One of the main goals of the electrochemical cell worksheet is to help you develop a systematic approach to solving problems in electrochemistry. It encourages you to break down complex problems into smaller, more manageable steps, and to apply the relevant principles and equations to each step. By doing so, you can gain a deeper understanding of the underlying concepts and develop the ability to tackle more advanced problems in the future.
Whether you are a student studying chemistry or a professional working in the field of electrochemistry, the electrochemical cell worksheet can serve as a valuable tool for learning and practice. By challenging yourself with the questions and actively seeking the answers, you can enhance your understanding of electrochemical cells and strengthen your problem-solving skills in this fascinating area of science.
The Electrochemical Cell Worksheet Answers
When working on the electrochemical cell worksheet, it is important to have a clear understanding of the concepts and principles behind electrochemical cells. By knowing the answers to the worksheet questions, students can demonstrate their understanding of topics such as redox reactions, cell potential, and the different components of an electrochemical cell.
The first question on the worksheet may ask students to identify the elements involved in a redox reaction. Here, students should be able to recognize the elements being oxidized and reduced, as well as the substances that act as oxidizing and reducing agents. They should also understand the concept of electron transfer and how it relates to the overall reaction.
A common question on the worksheet may also involve calculating the cell potential of an electrochemical cell. To answer this, students need to know the half-reactions and their standard reduction potentials. By applying the Nernst equation and considering the concentrations of the reactants and products, students can determine the cell potential and predict whether the reaction is spontaneous.
Furthermore, the worksheet may require students to identify the different components of an electrochemical cell, such as the anode and cathode. They should understand the role of each component in facilitating the electron transfer and how it contributes to the overall operation of the cell. Additionally, students may be asked to explain how the cell potential can be controlled by manipulating the concentrations of the reactants and products.
In conclusion, answering the electrochemical cell worksheet requires a solid understanding of redox reactions, cell potential, and the components of an electrochemical cell. By grasping these concepts and applying them to the specific questions, students can showcase their knowledge and comprehension of electrochemistry.
Understanding Electrochemical Cells
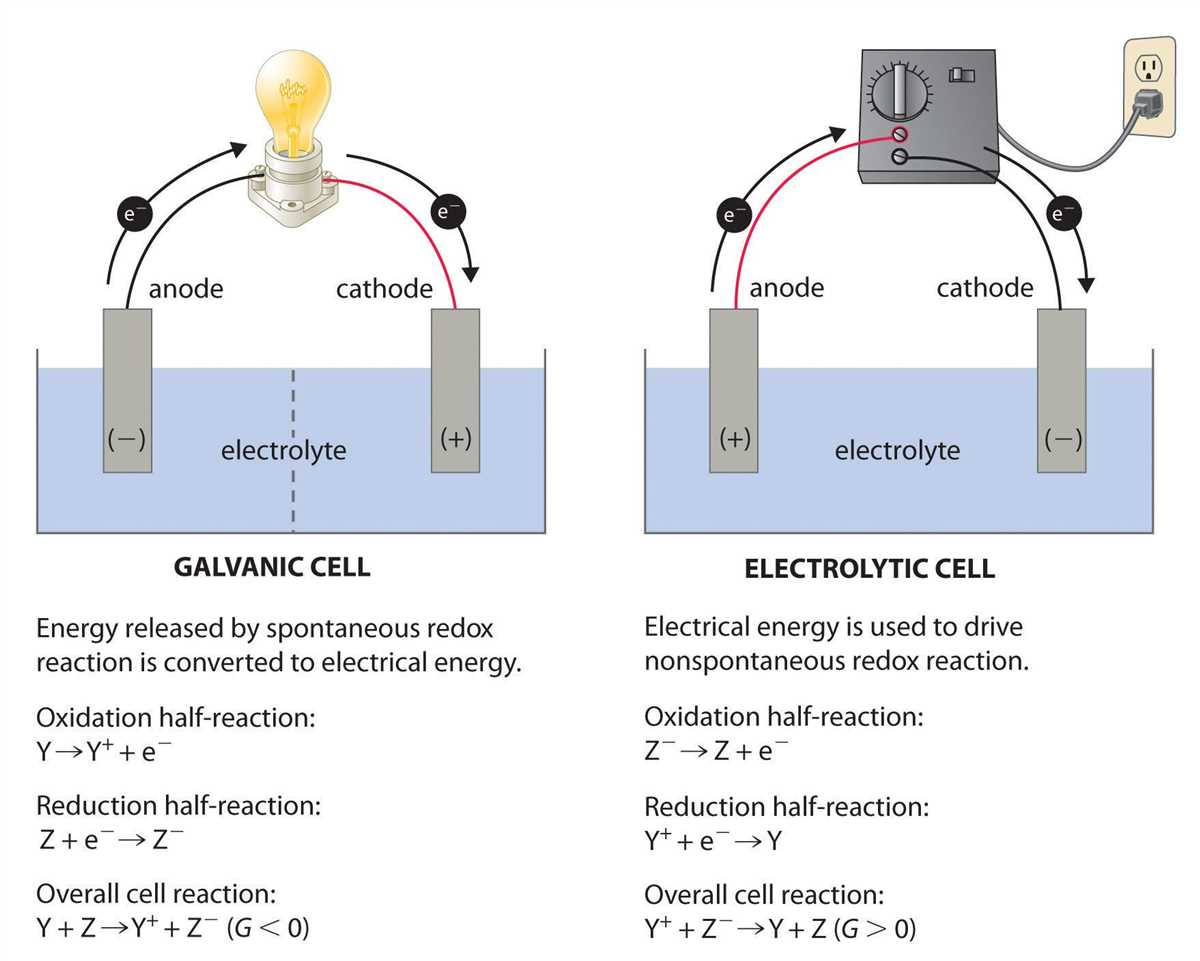
In the field of electrochemistry, electrochemical cells play a crucial role in understanding the principles of redox reactions and their applications. These cells are devices that convert chemical energy into electrical energy or vice versa, depending on the direction of the chemical reactions taking place within them. To comprehend the workings of electrochemical cells, it is essential to understand the structure and components that make up these systems.
An electrochemical cell consists of two electrodes, namely, the anode and the cathode, which are immersed in an electrolyte solution. The anode is the electrode where oxidation takes place, while the cathode is the electrode where reduction occurs. These two electrodes are connected externally by a wire, allowing the flow of electrons from the anode to the cathode.
Electrochemical cells can be further classified into two main types: galvanic (voltaic) cells and electrolytic cells. Galvanic cells are used to generate electrical energy through spontaneous redox reactions, while electrolytic cells are employed for non-spontaneous reactions, requiring an external energy source to drive the reaction. Both types of cells contain half-cells, which consist of the electrode and the electrolyte solution specific to that electrode.
Components of an Electrochemical Cell
1. Anode: The anode is where the oxidation half-reaction occurs, releasing electrons into the external circuit.
2. Cathode: The cathode is where the reduction half-reaction takes place, accepting the electrons from the external circuit.
3. Electrolyte Solution: The electrolyte solution facilitates the movement of ions between the electrodes, ensuring the completion of the redox reactions.
4. Salt Bridge: The salt bridge is a U-shaped tube filled with a concentrated electrolyte solution that connects the two half-cells. It allows the flow of ions to maintain electrical neutrality in the cell.
5. External Circuit: The external circuit is the pathway for electrons to flow from the anode to the cathode, allowing the conversion of chemical energy into electrical energy.
By understanding the components and functioning of electrochemical cells, scientists and engineers can harness their principles in various applications, such as batteries, fuel cells, and electroplating processes. Electrochemical cells are essential for powering electronic devices, storing renewable energy, and facilitating electrochemical reactions in various industries.
Components of an Electrochemical Cell
An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions. It consists of several components, each playing a crucial role in the overall functioning of the cell.
1. Electrodes: Electrodes are the conductive materials that come into direct contact with the electrolyte solution. There are two types of electrodes in an electrochemical cell: the anode and the cathode. The anode is the electrode where oxidation occurs, releasing electrons into the circuit. The cathode is the electrode where reduction occurs, accepting electrons from the circuit.
2. Electrolyte: The electrolyte is a solution or a substance that conducts electricity by dissociating into ions. It allows the flow of ions between the electrodes, completing the electrochemical circuit. The electrolyte can be an aqueous solution, a molten salt, or a gel-like substance depending on the type of electrochemical cell.
3. Salt Bridge: In some electrochemical cells, a salt bridge is used to maintain electrical neutrality and prevent the build-up of excessive charge. The salt bridge is typically filled with a gel-like substance containing an electrolyte. It allows the flow of ions between the two half-cells and balances the charges, ensuring the smooth operation of the cell.
4. External Circuit: The external circuit is the pathway for the flow of electrons from the anode to the cathode. It consists of a wire or a conducting material that connects the two electrodes and allows the electrical current to flow. The electrons released during oxidation at the anode travel through the external circuit to the cathode, where they are consumed during reduction.
5. Voltaic Cell: A voltaic cell, also known as a galvanic cell, is a type of electrochemical cell that generates electrical energy from a spontaneous redox reaction. It consists of two half-cells, each containing an electrode and an electrolyte, connected by a salt bridge or a porous barrier. The half-reactions at the anode and cathode generate a potential difference, resulting in the flow of electrons and the production of electrical energy.
Overall, the components of an electrochemical cell work together to facilitate the transfer of electrons and ions, enabling the conversion of chemical energy into electrical energy. Understanding the role of each component is essential for comprehending the functioning and applications of electrochemical cells in various fields, including batteries, fuel cells, and corrosion prevention.
Types of Electrochemical Cells
An electrochemical cell is a device that converts chemical energy into electrical energy or vice versa. There are several types of electrochemical cells, each with its own specific characteristics and applications.
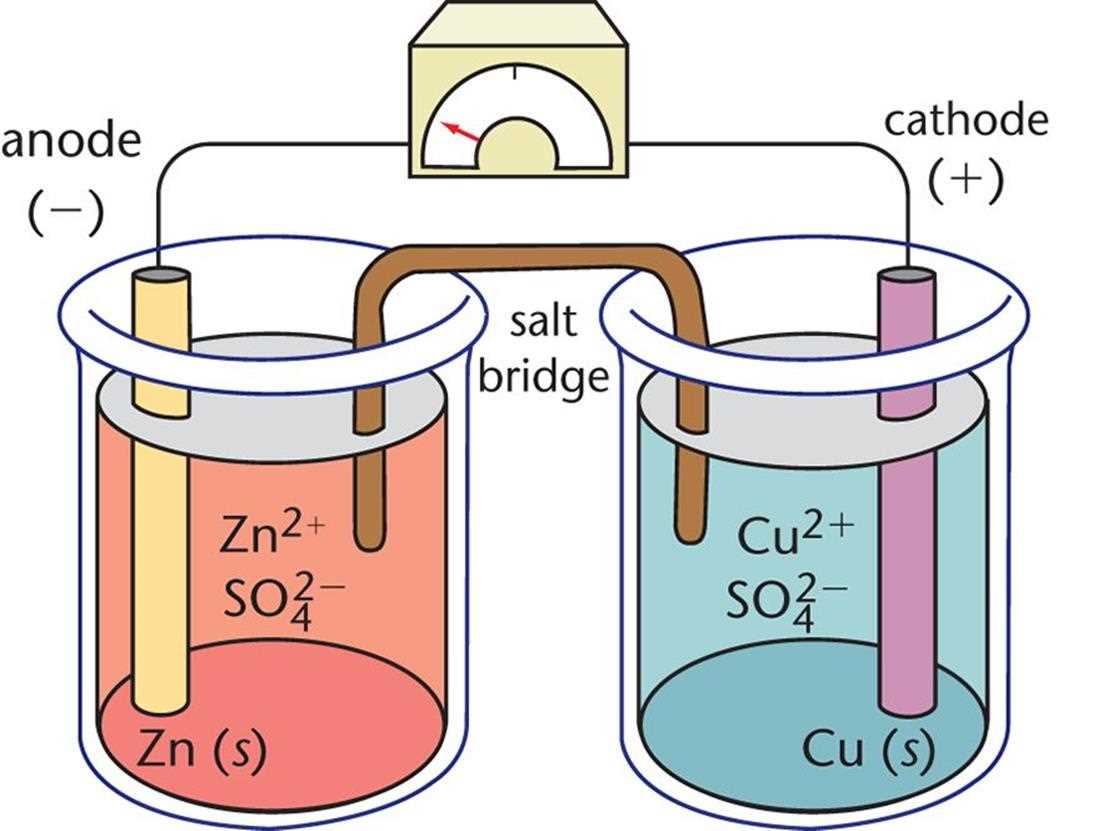
Galvanic or Voltaic Cells: This type of electrochemical cell is also known as a battery. It converts chemical energy into electrical energy through a spontaneous chemical reaction. Galvanic cells are commonly used in everyday devices such as flashlights and electronic gadgets. They consist of two half-cells, each containing an electrode and an electrolyte solution. When the two half-cells are connected by a conductive pathway, the electrochemical reaction occurs, generating an electric current.
- Example: The Daniell cell is a classic galvanic cell that consists of a zinc electrode immersed in a zinc sulfate solution and a copper electrode immersed in a copper sulfate solution. The chemical reaction between the zinc and copper electrodes produces an electric current.
Electrolytic Cells: Unlike galvanic cells, electrolytic cells convert electrical energy into chemical energy. They are commonly used in electroplating and electrolysis processes. Electrolytic cells consist of two electrodes (an anode and a cathode) immersed in an electrolyte solution. When an electric current is applied to the cell, a non-spontaneous chemical reaction occurs, allowing the deposition or dissolution of ions at the electrodes.
- Example: In the electrolysis of water, an electrolytic cell is used to split water molecules into hydrogen gas and oxygen gas. The anode attracts negatively charged ions (OH-) and releases oxygen gas, while the cathode attracts positively charged ions (H+) and releases hydrogen gas.
Fuel Cells: Fuel cells are electrochemical devices that continuously convert the chemical energy of a fuel directly into electrical energy. They are highly efficient and produce minimal pollution. Fuel cells consist of two electrodes (an anode and a cathode) separated by an electrolyte. The fuel, such as hydrogen or methanol, is supplied to the anode, while oxygen from the air is supplied to the cathode. The chemical reaction at the electrodes generates electricity.
- Example: The proton-exchange membrane fuel cell (PEMFC) is a type of fuel cell commonly used in transportation and portable applications. It utilizes a solid polymer electrolyte and operates at relatively low temperatures, making it suitable for a wide range of applications.
Electrochemical Cell Notation
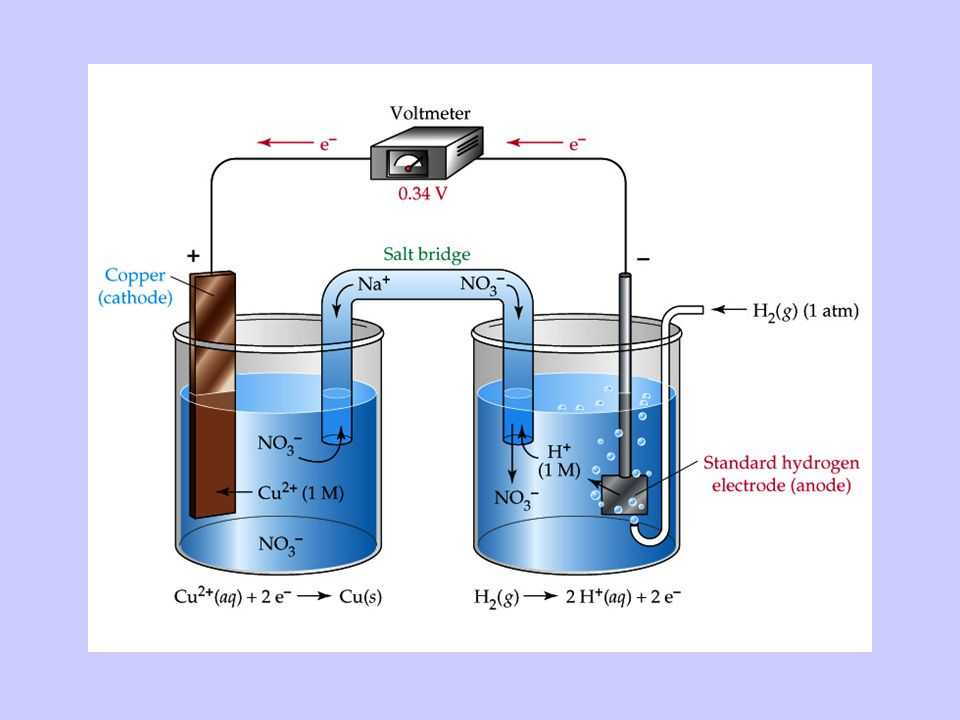
In electrochemistry, electrochemical cell notation is a shorthand representation of the reactions that occur in an electrochemical cell. It provides a concise way to describe the species involved in the cell and their respective states, as well as the direction of electron flow.
The notation consists of two half-reactions, one for the oxidation reaction occurring at the anode and one for the reduction reaction occurring at the cathode. Each half-reaction is written in the form “species + charge(s) → species + charge(s)”. The reactants and products are separated by a vertical line, indicating the phase boundary between the anode and cathode.
The notation also includes information about the electrolytes used in the cell. For example, if a salt bridge is used to maintain charge neutrality, it is represented by a double vertical line “| |”. If a porous barrier is used instead, it is represented by a single vertical line “|”.
To illustrate the electrochemical cell notation, let’s consider the Daniell cell, which consists of a zinc electrode immersed in a solution of zinc sulfate and a copper electrode immersed in a solution of copper sulfate. The oxidation half-reaction at the zinc electrode is written as “Zn(s) → Zn^2+(aq) + 2e^-” and the reduction half-reaction at the copper electrode is written as “Cu^2+(aq) + 2e^- → Cu(s)”. The cell notation for this Daniell cell would be:
- Anode (oxidation): Zn(s) → Zn^2+(aq) + 2e^-
- Cathode (reduction): Cu^2+(aq) + 2e^- → Cu(s)
- Cell notation: Zn(s) | Zn^2+(aq) || Cu^2+(aq) | Cu(s)
By using electrochemical cell notation, chemists and researchers can easily communicate the key details of an electrochemical cell setup and the reactions occurring within it. This notation is widely used in the study of electrochemical cells and is an essential tool for understanding and interpreting electrochemical processes.
Calculating Cell Potentials
The cell potential is a measure of the ability of an electrochemical cell to drive an electric current. It represents the maximum amount of work that can be done by the cell when it is connected to an external circuit. The cell potential can be calculated using the standard reduction potentials of the half-cell reactions involved in the cell. These reduction potentials can be found in tables, such as the Standard Reduction Potentials table.
To calculate the cell potential, we need to subtract the standard reduction potential of the anode from the standard reduction potential of the cathode. The anode is the electrode where oxidation occurs, while the cathode is the electrode where reduction occurs. The reduction potentials for the half-cell reactions are written as reduction reactions, so if we want to calculate the cell potential, we need to reverse the sign of the reduction potential for the anode reaction.
Once we have the standard reduction potentials for the anode and cathode reactions, we can simply subtract the reduction potential of the anode from the reduction potential of the cathode to obtain the cell potential. If the resulting value is positive, it indicates that the reaction is spontaneous and the cell potential is greater than zero. However, if the resulting value is negative, it indicates that the reaction is non-spontaneous and the cell potential is less than zero.
It is important to note that these calculations assume standard conditions, including a temperature of 25 degrees Celsius, a concentration of 1 mole per liter, and a pressure of 1 atmosphere. If the actual conditions differ from these standard conditions, additional calculations may be required to account for the differences. Additionally, it is important to be aware of the limitations of the standard reduction potentials, as they may not accurately represent the behavior of the cell under non-standard conditions.
Applications of Electrochemical Cells
Electrochemical cells have a wide range of practical applications in various fields. They are commonly used in batteries, fuel cells, and corrosion protection systems. Here are some notable applications:
Batteries
Electrochemical cells are at the heart of batteries, which are used to convert chemical energy into electrical energy. Batteries are used in everyday life to power electronic devices such as smartphones, laptops, and cameras. They are also used in transportation, providing power to electric vehicles. With advances in technology, the development of more efficient and long-lasting batteries is crucial for the transition to a cleaner and more sustainable energy future.
Fuel Cells
Fuel cells are electrochemical cells that generate electricity through the oxidation of a fuel, such as hydrogen or methanol. They have the advantage of producing electricity without the combustion of fossil fuels, resulting in lower emissions and improved energy efficiency. Fuel cells are used in a variety of applications, including transportation (e.g., hydrogen-fueled cars), portable power systems, and stationary power generation.
Corrosion Protection
Electrochemical cells are also employed in corrosion protection systems. By creating a galvanic cell, where a more reactive metal sacrifices itself to protect a less reactive metal, corrosion can be significantly reduced. This principle is utilized in various industries, such as marine, oil and gas, and infrastructure. For example, sacrificial anodes made of zinc or aluminum are often used to protect the hulls of ships or the underground pipelines from corrosion.
In conclusion, electrochemical cells have numerous applications that play a vital role in our everyday lives. From batteries to fuel cells and corrosion protection systems, these devices have revolutionized the way we store and use energy, while also contributing to sustainability and environmental protection.